
Home (Over-The-Counter) & Lab (Point-of-Care) Covid-19 Testing
With the rise (once again) of a Covid-19 variant - this time it's the "Omicron" variant - and the advance during the pandemic of testing, it’s time to provide some definition and perspective around the subject. This, like all medical areas, is complex.
We’ll provide some information available from sources like FDA, CDC, and other “reputable” sources, and include links to our source material. Like all health information, it’s always best to do some research on your own to back up what you get here or anywhere.
Covid-19 Basic Information
SARS CoV and SARS CoV-2 are sometimes used interchangeably, but they are not the same. SARS CoV was first detected in 2003. SARS CoV-2 is the current strain of Corona Virus, first detected in 2019. Mayo Clinic provides excellent information on symptoms, etc here: Mayo Clinic Covid-19 Information
Several Covid-19 vaccines (FDA Covid-19 Vaccine Information) have been created since this point. Vaccines have been seen to lower transmission rates, decrease the severity of symptoms, and decrease the death rate. However, even for those fully vaccinated and those who have also had booster shots, there are still cases of breakthrough transmission.
Nevertheless, at this time, the vast majority of those hospitalized with Covid-19 are among the unvaccinated.
In many areas and offices, testing is available as an added determiner of Covid-19 presence and helps to determine who should quarantine until symptoms are gone. This testing is now also becoming widely available to be done at home, so that you can take personal care using a home collection kit and know that you are positive - for isolation and treatment - or negative for Covid-19 and can return to work, school, normal life, etc.
Test Types
There are two basic types of testing available - diagnostic and antibody. (FDA Definitions)
A diagnostic test can show if you have an active coronavirus infection and should take steps to quarantine or isolate yourself from others.
Currently there are two types of diagnostic tests –
- Molecular (RT-PCR - “Reverse Transcription Polymerase Chain Reaction”) tests that detect the virus's genetic material, (Covid-19 PCR Test) and
- Antigen tests that detect specific proteins on the surface of the virus (Covid-19 Antigen Test)
Samples are typically collected with a nasal or throat swab, or saliva collected by spitting into a tube.
An antibody test looks for antibodies that are made by the immune system in response to a threat, such as a specific virus. Antibodies can help fight infections. Antibodies can take several days or weeks to develop after you have an infection and may stay in your blood for several weeks after recovery.
Over-The-Counter vs Point-Of-Care
When looking for these tests, you will find Over-The-Counter (OTC) and Point-of-Care (POC) products. Rapid Antigen test kits typically provide test results in 15 minutes or so. With both OTC and POC products, you can set up your own testing site, for example a school or office setting.
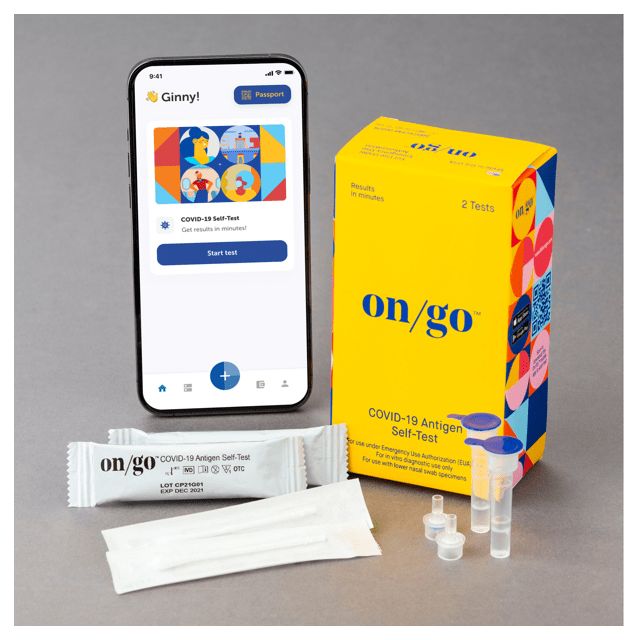
- OTC Tests (home test kits) have been certified by the FDA to be used at home (or anywhere, of course), by anyone (over 18, according to the FDA) - i.e. without the need for a certification or other special expertise. If you’re looking for a test you can administer yourself to determine whether or not you have Covid, then you’re looking for a diagnostic OTC test. These "home covid tests" are usable for both personal and office use - and, as noted, no training is needed to administer these.
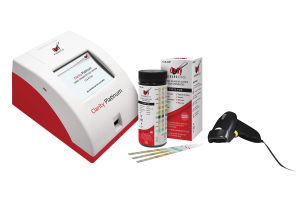
- POC Tests (clinical lab test kits) are used by medical offices and labs that have a government certification to perform these. (From CLIA Waivers Explained) All facilities in the United States that perform laboratory testing on human specimens for health assessment or the diagnosis, prevention, or treatment of disease are regulated under the Clinical Laboratory Improvement Amendments of 1988 (CLIA).These sites have a CLIA Certificate of Waiver (CLIA-Waived) and to order these test kits, the CLIA certificate must be on file with us.
Availability of Test Kits
At this time, the majority of tests available are diagnostic antigen tests, and UnimedUSA.com has several different kits available - both RT-PCR and antigen, although for practical purposes, availability of RT-PCR test kits is extremely limited, and antigen tests are significantly less constrained.
This is less a reflection of accuracy in any way, but more of manufacturing and ramp-up among the major manufacturers.
In the information block of each of these products on our site, you will usually find the accuracy level/percentage as well as other sensitivity/accuracy information.
You will also find that the size/unit of measure of each available product will vary.
The OTC (home covid-19 test) kits are available in units of 48 to 288 tests on up.
The POC/CLIA-Waived kits are available in units of 20 tests on up.
Final Thoughts:
We hope that whatever choice of Covid test kits you make, that the above is helpful information. If you have any thoughts, concerns, corrections, or suggestions on this blog post, please let us know. We want to be as accurate as possible, and provide both objective, and where noted, helpful subjective information for you to make an informed choice.
We’ve been around for over 43 years and focus on providing excellent customer service and competitive pricing in all areas of our medical and dental business.